Effect of Ozone on Bacteria
By Admin • April 24, 2020
Ozone not only destroys the bacterial cell, but it does it without leaving a residual effect.

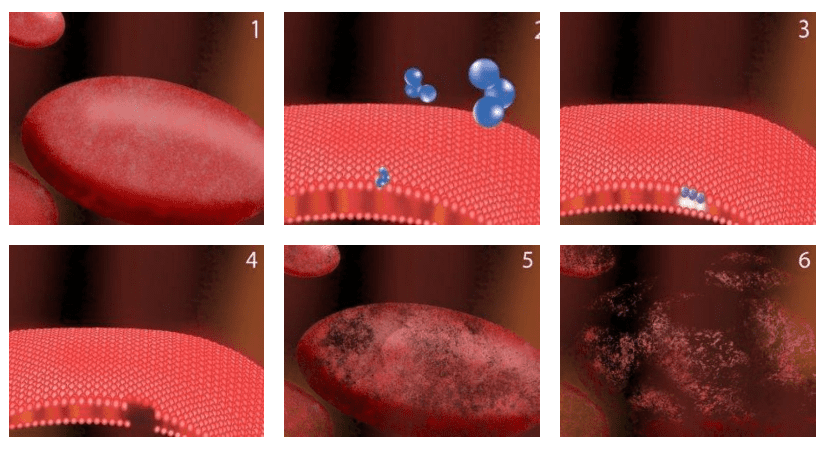
- A healthy bacillus bacterial cell (waiting to ruin your day)
- Zooming in closer, an ozone molecule (blue) comes into contact with the cell wall. The cell wall is vital to the bacteria because it ensures the organism can maintain its shape
- As ozone molecules make contact with the cell wall, a reaction called an oxidative burst occurs, creating a tiny hole in the cell wall.
- The newly created hole in the cell wall has injured the bacterium.
- The bacterium begins to loose its shape while ozone molecules continue creating holes in the cell wall.
- After thousands of ozone collisions over only a few seconds, the bacterial wall can no longer maintain its shape and the cell dies.
As a comparison based on 99.99% of bacterial concentration being killed and time taken, ozone is:
- 25 times more effective than HOCl (Hypochlorous Acid)
- 2,500 times more effective than OCl (Hypochlorite)
- 5,000 times more effective than NH2Cl (Chloramine)
Furthermore, ozone is at least ten times stronger than chlorine as a disinfectant. Chlorine reacts with meat forming highly toxic and carcinogenic compounds called THMs or tri-halomethanes – rendering the meat to a lesser quality product. THMs have also been implicated as carcinogens related to kidney, bladder and colon cancers. Chlorine also results in the production of chloroform, carbon tetrachloride and chloromethane. On the other hand, ozone does not leave any trace of residual product after its oxidative reaction.
Ozone not only destroys the bacterial cell, but it does it without leaving a residual effect.
- A healthy bacillus bacterial cell (waiting to ruin your day)
- Zooming in closer, an ozone molecule (blue) comes into contact with the cell wall. The cell wall is vital to the bacteria because it ensures the organism can maintain its shape
- As ozone molecules make contact with the cell wall, a reaction called an oxidative burst occurs, creating a tiny hole in the cell wall.
- The newly created hole in the cell wall has injured the bacterium.
- The bacterium begins to loose its shape while ozone molecules continue creating holes in the cell wall.
- After thousands of ozone collisions over only a few seconds, the bacterial wall can no longer maintain its shape and the cell dies.
As a comparison based on 99.99% of bacterial concentration being killed and time taken, ozone is:
- 25 times more effective than HOCl (Hypochlorous Acid)
- 2,500 times more effective than OCl (Hypochlorite)
- 5,000 times more effective than NH2Cl (Chloramine)
Furthermore, ozone is at least ten times stronger than chlorine as a disinfectant. Chlorine reacts with meat forming highly toxic and carcinogenic compounds called THMs or tri-halomethanes – rendering the meat to a lesser quality product. THMs have also been implicated as carcinogens related to kidney, bladder and colon cancers. Chlorine also results in the production of chloroform, carbon tetrachloride and chloromethane. On the other hand, ozone does not leave any trace of residual product after its oxidative reaction.